By:
Jack Roe
Vice President & General Manager, Labeling Systems LLC
Government mandates to serialize pharmaceutical products are increa
The California State Board of Pharmacy is requiring that 50% of each manufacturer’s drug products be serialized by January 1, 2015, with the remaining 50% serialized by January 1, 2016. Turkey has required that all drugs be serialized since January 1, 2010. Brazil will require serialization beginning in January 2011. Italy, Belgium, Greece and France all have
programs in place.
There are two predominant drivers behind these serialization mandates:
• Fighting grey market diversion and counterfeiting
• Aid in government reimbursement programs
Details vary from region to region, but in general all mandates require marking the smallest unit of sale with lot and expiration information, as well as a serial number, creating a unique license plate for each unit.
To facilitate 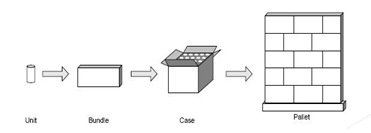
tracking units
through the supply
chain, units are
aggregated into
bundles, bundles
into cases and
cases onto pallets, with the parent child relationships maintained in a database.
License plate information for units, bundles, cases and pallets is typically marked on the product in human readable form, as well as being encoded in a 2D code and/or an RFID tag.
Although RFID was central to the discussion just a few years ago, it is becoming less prevalent because of the cost of tags and concerns about meeting the pharmaceutical industry’s six sigma quality requirement. 2D codes are becoming the more common choice for encoding units and aggregations.
As these concerns with RFID are addressed, the advantages of the non‐line‐of‐sight nature of RFID and the ability to “x‐ray scan” a case to read all units in the case at once, more programs will embrace the error reduction and labor savings that RFID affords.
Although pressure sensitive labels, with or without RFID tags, are the most common form of marking for bundles, cases and pallets, laser marking and ink jet coding are more common for units, because of space limitations and throughput speed requirements.
The lion’s share of the effort required to implement Track & Trace is spent on data management. Tracking the chain of custody of each unit from manufacturing, through distribution, up to the point where it is administered is a monumental task.
The data security to ensure that each unit can be authenticated adds to the complexity.
The first critical piece of the chain occurs right on the packaging line. Precise and consistent product handling, marking, inspection and data gathering are required to introduce accurate data into the front end of the system.
Expertise with product handling, labeling and other marking technologies, barcode scanning, machine vision, RFID, controls and software interfaces are a necessity to make a packaging line track & trace ready.
Disciplined project management to coordinate activities of packaging equipment suppliers, vision manufacturers, RFID experts, software vendors and the end user is also essential to a project’s success.
About Labeling Systems:
Labeling Systems, a division of Pro Mach designs and manufactures a complete line of heavy duty pressure sensitive labeling equipment. Since inception, LSI has been building machines that
stand up to the abuses that a 24/7 production line demands.
LSI’s headquarters are located in Oakland, NJ.
For more information call (201) 405‐0767 or visit www.labelingsystems.com.




Comments
Post a Comment